Research
In the Sipe laboratory, we study the interactions between neurons and glia ('non-neuronal cells') in sensory processing, arousal, stress, and addiction. This research can be grouped into three foci:
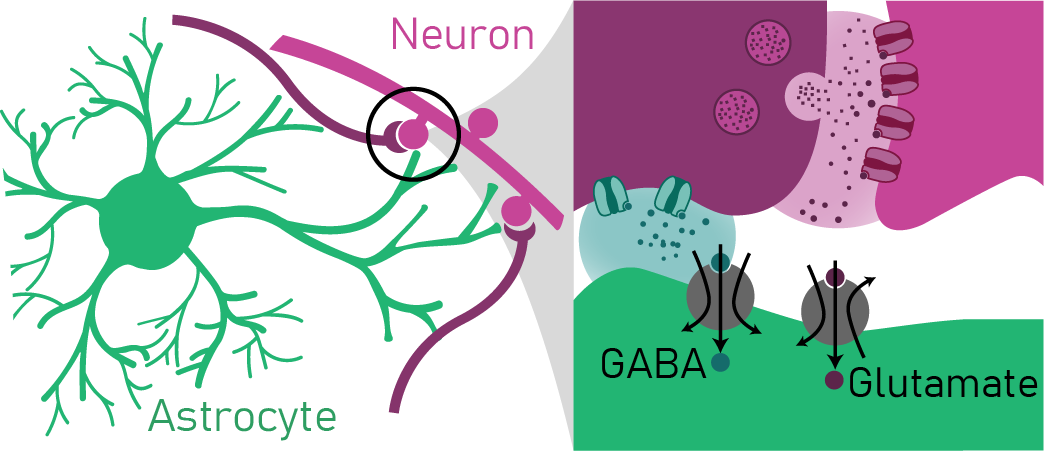
Focus 1: Astrocyte mechanisms modulating inhibition of neuronal networks
Astrocytes are one class of glia that serve critical roles in brain homeostasis and plasticity. Astrocytes are able to influence neuronal excitation and inhibition through a variety of mechanisms including ionic gradient maintenance, neurotransmitter uptake, metabolic buffering, and neuroactive substance release. Our laboratory is particularly interested in how astrocytes influence inhibition through transporter uptake of the inhibitory neurotransmitter GABA as well as the release of endozepines (molecules that potentiate inhibition). These interests relate to neuropsychiatric disorders characterized by imbalance of neuronal excitation/inhibition such as anxiety, depression, schizophrenia, and autism. Focus 2: Neuromodulation of astrocyte-neuron networks during shifts in arousal
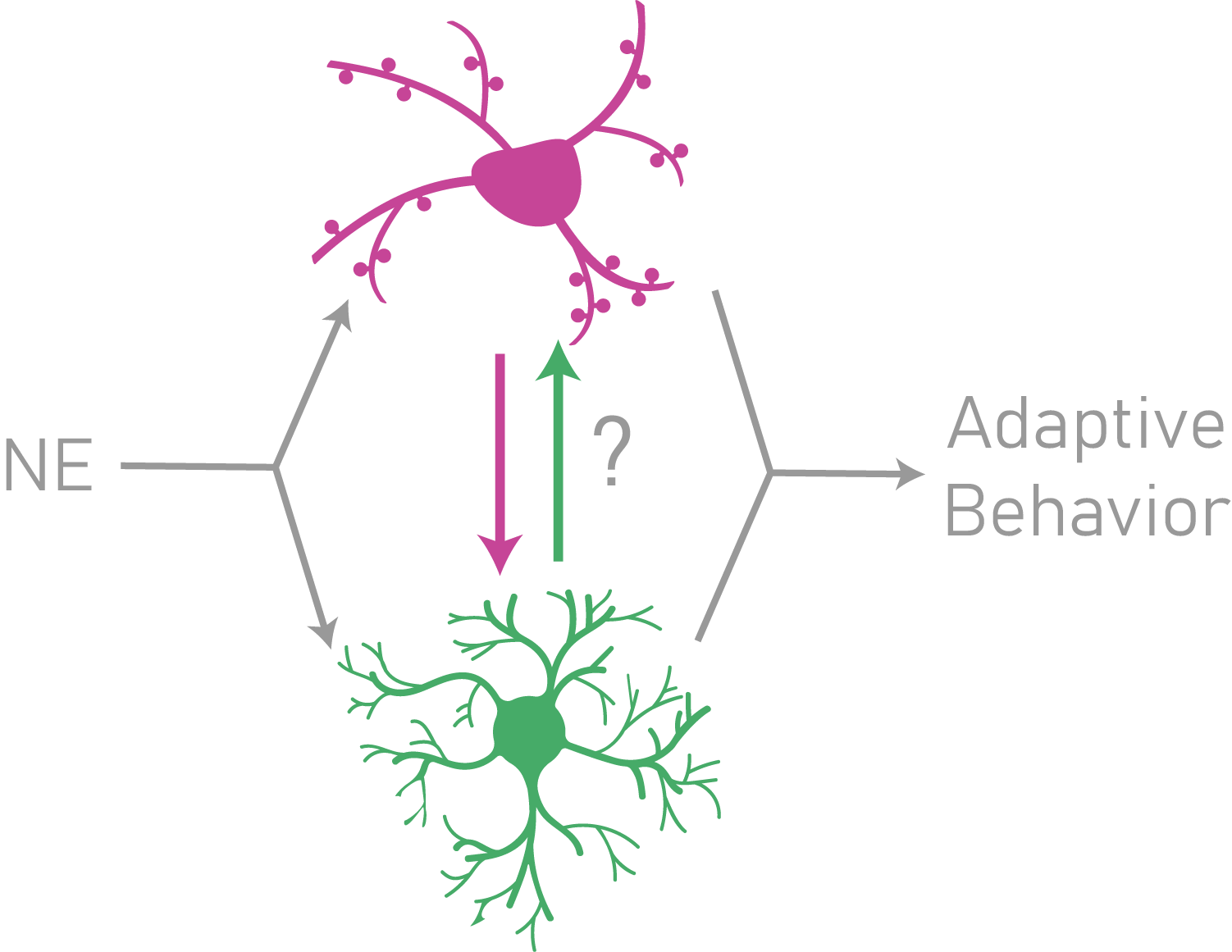
Neuromodulation during arousal shifts fundamentally shapes brain states and is critical for sensory processing and adaptive decision-making. Therefore, studying the molecular and cellular mechanisms that drive shifts in arousal is crucial for understanding maladaptive arousal in neuropsychiatric disorders such as anxiety and post-traumatic stress disorder (i.e. PTSD). Despite the breadth of work studying neuromodulation from a neuronal perspective, relatively little is known about neuromodulation of astrocytes and their subsequent influence over neuronal circuits. Our laboratory is specifically interested in the effects of the neuromodulator, norepinephrine (NE), on both astrocytes and neurons, and how these effects shape sensory processing and decision-making in dynamic environments. Focus 3: Astrocyte mechanisms underlying the development of addiction
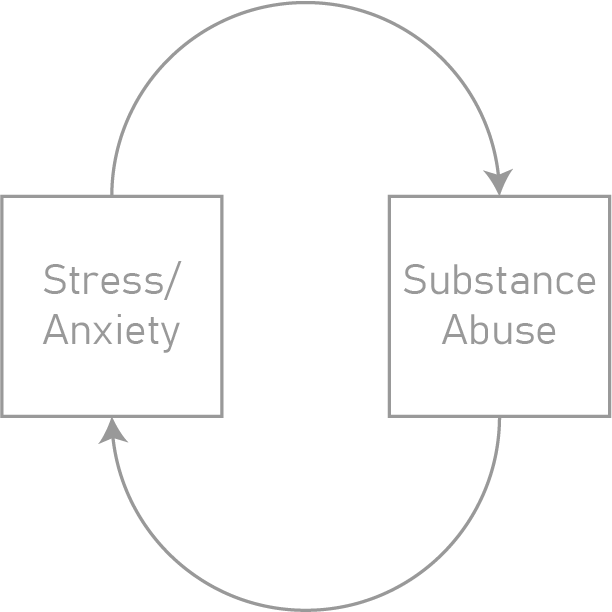
Bidirectional signaling between astrocytes and neurons occurs across diverse spatiotemporal scales from synaptic transmission on the order of milliseconds to synaptic plasticity on the order of days. Astrocyte-neuron signaling is therefore a potential mechanism by which acute substance abuse transforms into addiction. Our laboratory is specifically interested in how astrocyte-neuron physiology contributes to the development of binge-like alcohol consumption. We aim to bridge the timescales of signaling during active alcohol consumption to synaptic plasticity across days. We are also interested in linking maladaptive arousal (i.e. stress, anxiety) with predisposition to developing substance abuse.